The Science Behind the Nobel Prize
The pioneering work of Mary Brunkow and Fred Ramsdell began with a mysterious mutant mouse known as “scurfy,” leading them to identify the FOXP3 gene and unlock how regulatory T cells prevent autoimmune disease — discoveries that now point to new treatments in cancer and autoimmunity.

By Rachel Tompa, PhD, contributing writer – special to ISB
It all started with a mouse called scurfy.
In the mid-1990s, Dr. Mary Brunkow was on a gene hunt, one that would ultimately lead to defining and characterizing a new class of immune cells. In 1994, the geneticist/molecular biologist took a position at Darwin Molecular, a Seattle-area biotech company co-founded by ISB’s Dr. Leroy Hood and led by Dr. David Galas. At the time, the Human Genome Project was running at full speed and catalyzing novel genomics-related technologies and approaches. Brunkow and her team aimed to apply and further advance these genomics tools to demonstrate that determining the molecular basis of genetic diseases with relevant clinical features could lead to the identification of novel drug targets.
Brunkow and her co-Nobel Prize winner, immunologist Dr. Fred Ramsdell, tackled an immune mystery: The mutant mouse strain known as scurfy. These mice showed hallmarks of severe autoimmune disease – their immune systems were attacking their own bodies.
The scurfy strain arose in 1949, the result of a spontaneous mutation in the Oak Ridge National Laboratory’s mouse collection. This “Mouse House” was originally established to study the genetic effects of radiation exposure. The Oak Ridge scientists named the strain scurfy for its characteristic scaly skin. The mice also had enlarged lymph nodes and spleens, and they died three weeks after birth.
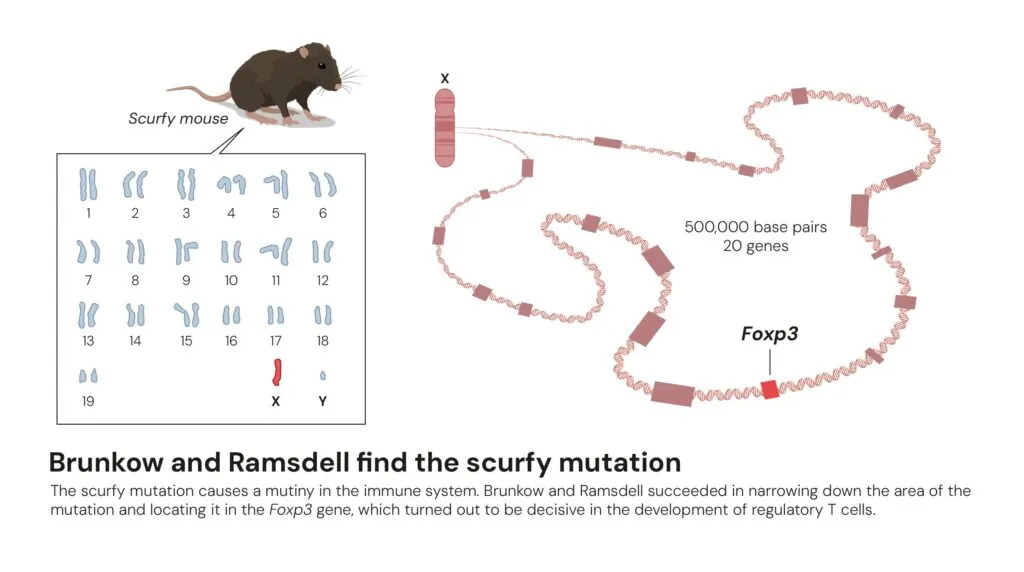
The Oak Ridge team had maintained the scurfy strain for more than 45 years by the time Brunkow and Ramsdell began to study it. Only male mice showed symptoms, suggesting that the mutation was somewhere on the X chromosome. Unlike females, which carry one normal and one mutant version of the X chromosome, scurfy male mice have only the mutant X chromosome. This was one of the first X-linked diseases described in mice, but the gene that caused it remained unknown.
Although today entire genomes can be sequenced in hours, sequencing techniques 30 years ago were more laborious: It took 13 years to sequence the first human genome. Through the painstaking work of developing and merging genetic and physical chromosome maps, Brunkow and her team narrowed the scurfy region on the X chromosome to a region that contained just 20 genes. They then sequenced each of those genes in scurfy mice and in healthy animals, and, in the 20th gene they studied, they finally found the genetic cause of the disease – two additional DNA letters inserted within the sequence of a never-before-described gene, which came to be known as FOXP3. That tiny change in the gene’s sequence resulted in an incomplete and non-functional FOXP3 protein.
Based on FOXP3’s genetic sequence, the scientists could tell that it belonged to a family of similar genes, all of which code for proteins that bind to DNA and regulate the expression of other genes. Brunkow, Ramsdell, and their collaborators at the University of Washington and Oregon Health & Science University went on to show that a rare human autoimmune disorder called immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, or IPEX, results from mutations in the human FOXP3 gene. The scientists published three seminal studies in 2001 in the journal Nature Genetics describing their discovery of FOXP3 and its connection to human disease.
Although the researchers knew scurfy and IPEX were autoimmune diseases involving immune cells known as T cells, it was unclear at the time how FOXP3 acts to keep the immune system functioning normally. The answer to that question would partially solve an even larger mystery: how our immune systems kill harmful intruders like viruses but refrain from attacking our own bodies.
T cells have a wide variety of roles, and an even wider variety of different proteins they recognize and respond to. To distinguish our own proteins from foreign ones, T cells go through training in the thymus gland. Here, T cells that recognize the body’s own proteins are eliminated. But in the 1980s, scientists proposed that there must be another system to eliminate rogue T cells that slip through the thymus’s training ground.
Dr. Shimon Sakaguchi, the third Nobel Prize co-winner, found that system, and it turned out to be a subclass of T cells themselves. These “regulatory T cells” patrol the body, looking for other T cells that recognize the body’s own proteins and eliminating them.
Ramsdell’s group, Sakaguchi’s group, and Dr. Alexander Rudensky’s group at the University of Washington found that FOXP3 is essential for the development of regulatory T cells. Without FOXP3, these cells can’t develop. Importantly, the FOXP3 protein provided a molecular “handle” for scientists to identify and study regulatory T cells – no other protein unique to this class of T cells had been found up until then.
Since the trio’s initial discoveries in the 1990s, scientists have gone on to uncover many important aspects of regulatory T cells and their importance in the body. These cells are a key part of how tumors evade detection by the rest of the immune system, and cancer immunotherapies under development are testing whether removing regulatory T cells in a tumor could allow the body’s immune system to find and eliminate the cancer cells.
While no therapies targeting regulatory T cells are available yet, several promising treatment avenues are in early-phase clinical trials, including harnessing these cells’ power to improve organ transplantation and to treat autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease.
Related publications
Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001:27:68-73.
Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow M. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001:27:18-20.
Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001:27:20-21.
Fontenot J, Gavin M, Rudensky. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003: 4: 330-336.
Khattri R, Cox T. Yasayko S-A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003:4:337-342.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003:299:1057-61.